How are genetically engineered (GE) crops different from those that are not genetically engineered?
The short answer is that the genes used in genetically engineered crops are often not from an organism that is similar to the crop. The GE genes may introduce unexpected problems.
A difference in the source of the genes used
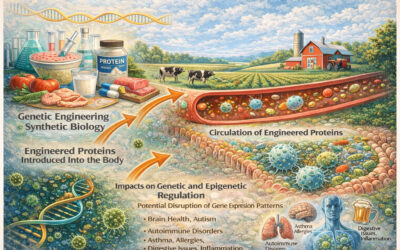
GE crops have had foreign DNA added to the original DNA in each of their cells. This foreign DNA could be from a different plant, an animal, a virus or bacterium, or wholly synthesized in a lab. It could come from an extinct organism, or even be extracted from an old leather shoe! This new DNA, for the majority of the crops under cultivation, results in the plants making their own insecticide or being tolerant to specific herbicides. Most GE organisms also contain additional foreign gene(s) introduced so that scientists can track whether the introduction was successful. For example, some GE crops contain, in each cell throughout the organism, a gene responsible for conferring resistance to an antibiotic. These foreign genes are often “turned on” continuously and at high levels during the life course of the GE plants that contain them. These genes are used by the plant as the blueprint for the production of foreign proteins with functions previously unavailable to the type of plant “transformed” through the genetic engineering process.
A difference in the way GE food crops are grown
There are also differences in the way GE foods are grown, as compared with non-GE crops. A crop with the Roundup Ready® gene makes a protein that allows farmers to spray Roundup® on the crop without killing the crop. Roundup, which contains glyphosate as the active ingredient, is an herbicide used to kill weeds that would compete with the crop. As Roundup becomes incorporated into the plant’s cells, the consumer can, however, be exposed to the herbicide when consuming these foods. When not using a Roundup Ready crop, farmers have to use different methods to prevent weed infestations. Organic farmers use mulching, tilling, cover crops, plastic ground covers and other means to minimize weed problems. Non-organic farmers not using GE crops use herbicides judiciously so as not to harm their crops.
In the US, 99% of GE crops currently cultivated were developed for two reasons:[ref]USDA Economic Research Service: Adoption of Genetically Engineered Crops in the U.S: Recent Trends in GE Adoption, http://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx#.VDljjLvVLRc[/ref]
– to allow farmers to spray herbicides on their crops to kill weeds without killing the crops themselves, i.e., by planting “Roundup-Ready” seeds
– so the crop itself makes an insecticide called a Bt toxin.
These so-called Bt crops continually produce Bt toxin in the plant itself. Such GE plants are actually registered as pesticides by the EPA.
The remaining 1% of GE crops currently commercially available were developed for other reasons, such as resistance to a virus (i.e. GE papaya and GE squash). Some GE foods not yet on the market include salmon engineered to grow faster, apples engineered to prevent browning so they can be sold pre-sliced, and so-called “Golden” rice, which is engineered to produce the precursor of Vitamin A and is being developed for areas of the world where vitamin A deficiency is common.
Haven’t regulatory government agencies found levels of Roundup pesticide used on GE food to be safe?
We do have several studies arguing for the safety of the present levels of Roundup (glyphosate), and the FDA has recently raised allowable levels of glyphosate in our food.
However, the FDA does not actually perform or rely on independent testing of the foods it reviews for approval. In a legal document, FDA oversight is described thus:
“Ultimately, it is the responsibility of the producer of a new food to evaluate the safety of the food and assure that the safety requirement of section 402(a)(1) of the act is met.”[ref]Guidance to Industry for Foods Derived from New Plant Varieties, Section V (B) & (C), http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/
Biotechnology/ucm096095.htm[/ref]
Some authors even conclude that:
“The FDA approach can be understood as the result of having a dual mission. In addition to its mission to protect food safety, the FDA was charged with promotion of the biotech industry.”[ref]Boston College Law Review, 44 Rev. 733 (2003), Risk and Regulation: U.S. Regulatory Policy on Genetically Modified Food and Agriculture, http://lawdigitalcommons.bc.edu/bclr/vol44/iss3/2, Marden, E.[/ref]
An additional concern is that members of FDA advisory panels may be influenced by financial conflicts of interest. A recent study shows that members who served on advisory boards solely for the sponsoring firm were 5 times more likely to vote in favor of the sponsor [ref]Milbank Quarterly, 92: 446–470 (2014), Revisiting Financial Conflicts of Interest in FDA Advisory Committees, Pham-Kanter, G.[/ref].
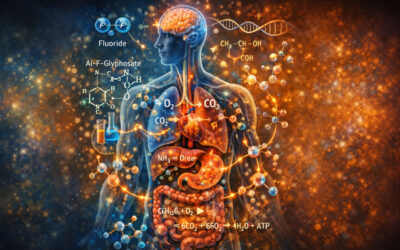
From a medical perspective, significant caution is warranted. We can look to history as our guide. There have been many instances when regulatory agencies argued for the safety of a substance, even though basic science raised concerns. Harm was done to large portions of the population until the substance was finally banned or highly regulated. Lead and tobacco are prime examples, and the story is repeated with other pesticides and industrial chemicals, including chlordane[ref]http://ecommons.cornell.edu/bitstream/handle/1813/14376/CE.4_chlordane.pdf?sequence=2&isAllowed=y[/ref], polycarbonated biphenyls (PCBs) [ref]http://en.wikipedia.org/wiki/Polychlorinated_biphenyl[/ref] and most recently BPA[ref]http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm064437.htm[/ref]
Lead was once considered safe at the levels found in paint and gasoline. For childhood lead, the blood level of concern, which was originally set at 40 μg/dL, was lowered to 25 μg/dL and then to 10 μg/dL in 1991 [ref]Am J Public Health, 2003 August; 93(8): 1253-126, Should the Centers for Disease Control and Prevention’s Childhood Lead Poisoning Intervention Level Be Lowered? Bernard SM[/ref]. The safe lead level was lowered further in 2012 [ref]New York Times: C.D.C. Lowers Recommended Lead-Level Limits in Children, May 16, 2012, Hartocollis, A.[/ref] after an additional decade of debate. (The pace of change can be very slow.) Now we suspect that there is no safe blood lead level, and that elevated blood lead can lead to severe neurobehavioral harm (learning disabilities and serious behavior issues), cardiovascular problems, and bone, reproductive and renal disease [ref]Agency for Toxic Substances and Disease Registry: Lead Toxicity: What are the Physiologic Effects of Lead Exposure, http://www.atsdr.cdc.gov/csem/csem.asp?csem=7&po=10[/ref].
There is a rapidly growing body of research linking the herbicide Roundup (and its active ingredient glyphosate) to a number of harmful effects on a variety of organisms.
We examine these in detail in the section named “Glyphosate”. We make the case here, and here, that these studies provide biological plausibility of harmful effects, especially with regard to the rising medical illnesses we are facing. (link to Top5pediatric and Pediatric Perspectives). We are especially concerned as the use of glyphosate continues to rise significantly, from 1.5 million pounds in 1999 to about 90 million pounds in 2011 [ref]Washington State University College of Agricultural, Human and Natural Resource Sciences. Oct 1, 2012, Pesticide Use Rises as Herbicide-Resistant Weeds Undermine Performance of Major GE Crops, New Study Shows, Benbrook, C.[/ref].
Do genetically engineered foods affect health?
In order to adequately answer this question, ideally we would need controlled, long-term feeding studies on humans.
An additional complication is that there are many types of GE plants. Some are protected against herbicides (Roundup Ready®), some make insecticides called Bt toxins, some have both, many have antibiotic-resistance genes and other express additional traits. Each individual GE crop should be tested for unintended, unexpected changes that might affect its safety.
Unfortunately, proper long-term animal feeding studies have not been conducted with any GE crops. These types of studies are those in which the animals are divided into two groups receiving different foods, and these foods should be as identical as possible except for the genetically engineered trait. In the experiment described below, GE corn MON810 was used; this is corn genetically modified to make the particular Bt toxin/insecticide Cry1Ab. The parent corn of MON810 (the corn variety into which the Bt gene was inserted to create MON810) which is not genetically modified and does not make the insecticide, was used in the feed fed to the control rats in this experiment [ref]Food and Nutrition Sciences, 2014, 5, 185-195, Feeding Study with Bt Corn (MON810: Ajeeb YG) on Rats: Biochemical Analysis and Liver Histopathology, Abdo EM, Barbary OM, Shaltout OE[/ref].
The two groups of rats consumed a diet containing either 30% GE corn, or 30% non-GE corn. (The authors of this study also had a group of rats on an additional “control diet” but they do not give details as to the composition of this diet, so we will not use that group in our analysis of their data.)
Their first finding is that the two types of corn are not identical: proteins, carbohydrates, fat content, and other parameters they measured, differed between the two types of feed. This difference presumably resulted from the genetic modification.
After 45 days, one group of rats was sacrificed, and the authors found differences in the weight of their organs. For male rats, the animals eating GE corn had enlarged spleens, and for female rats, enlarged livers. A second group was sacrificed after 3 months. In this group, the males on GE feed had enlarged spleens, livers and kidneys. The females had enlarged livers and spleens.
These researchers went on to analyze blood taken from these rats and looked at their livers under a microscope. They found several differences between the groups. Under the microscope, the enlarged livers showed damage. The blood tests agreed with the visual evidence, revealing increased levels of liver enzymes, a sign of liver damage (when liver cells rupture, liver enzyme levels are higher).
Relevance to human health
GE corn cultivation has increased dramatically in the last 20 years [ref]USDA Economic Research Service, Adoption of Genetically Engineered Crops in the U.S.: Recent Trends in GE Adoption[/ref]. If the effects reported in the study described above were relevant to human health, depending on how quickly harm similar to that observed in rats would occur in humans, we would expect to see an increase in liver damage among humans too.
In fact, we do.
There are two liver diseases presently on the rise: they are called non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) and they involve liver damage. In these diseases, which can progress to liver failure or cancer, the liver cells become damaged, and levels of liver enzymes rise. These diseases are related to lifestyle, especially the consumption of fast foods, most of which contain genetically engineered ingredients. No one has explicitly connected these diseases with corn consumption, or with any of the Bt toxins. However, NAFLD has doubled in adolescents, for example, over the last 20 years [ref]J Pediatr. 2013 Mar;162(3):496-500, Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010, Welsh JA, Karpen S, Vos MB[/ref]. The authors of the study specifically point out that the rise in obesity is not enough to explain the trend.
We cannot conclude from the one study of MON810 that GE corn is harmful to humans. A human feeding study would have to be undertaken. However, such a study poses enormous logistical difficulties and we are concerned that there are no reports of one being under way.
Without such a study, we cannot be assured that any GE corn is safe for human consumption.
What are the arguments for and against labeling of genetically engineered ingredients in our food?
When polled, the vast majority of Americans say they would like to know which foods contain genetically engineered ingredients [ref]New York Times: Science; Strong Support for Labeling Modified Foods, By Allison Kopicki, July 2013[/ref]. Proponents of labeling laws would like the right to choose when and if to consume GE ingredients. Currently in the Unites States, the only way to choose to not consume GE ingredients is to eat only organic foods. Reasons for opposing GE ingredients range from health concerns to environmental reasons, to preference for traditional ingredients, to freedom of choice. It is obviously more difficult to avoid GE foods when they are not labeled, and 64 other nations already label foods with GE ingredients [ref]Genetically Engineered Food Safety Labeling Laws, Center for Food Safety (2012), http://www.centerforfoodsafety.org/ge-map/[/ref]. Another argument for labeling is that it makes epidemiological studies of GE food consumption possible because participants can state whether they buy foods containing GE ingredients or not. Funders of campaigns to label GE foods include the Organic Consumers’ Association, Dr. Joseph Mercola, groups of physicians and scientists focused on environmental concerns, and hundreds of individual donors to the labeling campaign.
Opponents of labeling foods that contain GE ingredients believe that the public is unfairly biased against GMOs. They state that many members of the public are easily frightened because they have a poor grasp of science. Many manufacturers of foods with GE ingredients argue that these foods are perfectly safe, and they do not want to run the risk of having consumers avoid their products. Some authors also argue that genetic engineering is a crucial technology that offers important potential for developing healthier foods and plants that can grow with greater yields, less pesticide and less water. They voice concern that opposition to the GE foods presently available would undermine future research into other GE organisms by weakening public support for genetic modification technology. Funders opposing GMO labeling laws include Monsanto, Bayer Crop-Science (makers of genetically engineered seeds and the pesticides used on GE crops), the Grocery Manufacturers’ Association, associations of growers of corn, soy, cotton, canola and other plants that are almost entirely genetically engineered, and many others.
We think that the assertion by industry that consumers might avoid GE food ingredients is a poor reason to deny American citizens the right to know which of their foods contain such ingredients. This denial of the right to know is especially misguided in light of (1) the dearth of long-term safety studies on GE crops, and (2) the loopholes in U.S. regulation that can let some GE crops be commercialized without undergoing any pre-market regulation at all.