Rising rates of childhood diseases
About 43% of US children (~14 million out of 32 million) have at least 1 of 20 different chronic health conditions.[1] Even more worrisome is that the incidence rates of the following diseases and conditions have shown significant increases in the last 20 years, but with no clear explanations: cancer,[2] asthma and allergies[3] – including allergies requiring hospitalization[4] – Type 1 diabetes,[5] inflammatory bowel disease,[6] behavioral and learning disabilities,[7] and (although it is somewhat debated) autism spectrum disorder.[8]
When the rate of a disease increases over a period of a few years, changing nutritional and environmental influences are a likely cause since human genes do not change that quickly. Thus, one or more external factors likely explain the increased numbers of cases of the childhood diseases mentioned above. All the factors that have changed in children’s lives should be investigated, especially those resulting in pervasive, near-universal exposures. It would be prudent to consider whether there is a plausible biological link between GE foods and certain childhood diseases, in at least some children.
The first thing to understand about the childhood illnesses named above is that in nearly all individuals, there isn’t one single factor that can be pinpointed as their cause. For example, some children get asthma from a combination of air pollution, stress, and nutritional deficiencies. Others may get it from having had smoking parents and early exposure to substances that alter their gut bacteria, such as antibiotics. So, for instance, a food contaminant that disrupts human gut bacteria could be one of several cumulative influences on children’s immune system.
A change in our children’s diet
It has been estimated that 70 to 80% of the processed and manufactured foods available in a conventional grocery store contain one or more ingredients derived from genetically engineered (GE) organisms (also known as “GMOs”).[9] The most common GE-derived ingredients include corn, soybean, and canola cooking oils, high-fructose corn syrup, a variety of meals and flours, and dozens of other ingredients designed to increase protein content, alter cooking and baking properties, or add other attributes to a given food product.
Children are exposed to GE foods in soy infant formula, soymilk, corn chips, and corn-based cereals, as well as to GE ingredients in most processed foods that contain soy- and corn-derived ingredients. In addition, they consume the products of animals that have been fed GE crops. The GE industry claims that the novel proteins in GE foods consumed by animals are broken down in the animals’ digestive tracts and that they are therefore not present in the foods children eat. Contrary to these claims, there are studies that have identified intact GE proteins in processed and manufactured foods and in animal products.[10] Despite these findings, the government, industry, and independent scientists have made little effort to track the pathways through which GE proteins are broken down and to assess the possible toxicity or allergenicity of the breakdown products.
We have a responsibility to ensure that GE foods are not causing unintended, unexpected health effects. Over the last two decades, GE varieties of corn, soybeans, and cotton cultivated in the US have steadily increased. By 2014, the vast majority of these three crops were genetically engineered (see graph above); for example, 95% of the acres of soybeans were planted in GE soybean varieties.[11] GE wheat varieties are not commercially available at this time.
Not only are we consuming these GE crops, but they are fed to animals as well, which impacts the nutrient composition, and possibly safety, of meat and poultry, eggs, milk and dairy products, and farmed fish. In the case of foods from Roundup Ready® crops, glyphosate residue may also be present.
The role of glyphosate
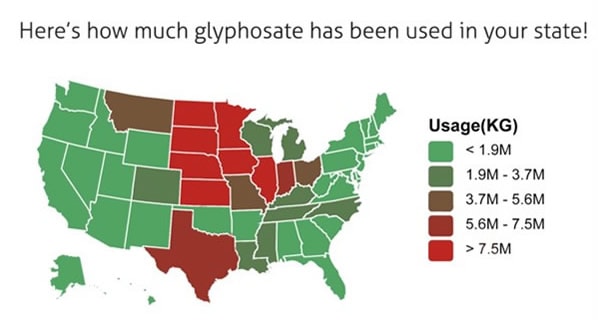
Most GE food crops have been engineered to resist the herbicide glyphosate, the active ingredient in Roundup®. The amount of glyphosate used on farmland in the US has increased rapidly over the last 15 years, from 85-90 million pounds in 2001 to 180-185 million pounds in 2007[12] and around 250 million in 2015[13]. It is also known that formulated herbicide products that contain both glyphosate and a mix of so-called “inert” ingredients (mostly adjuvants and surfactants) are more toxic than glyphosate by itself, and that the unique mix of ingredients in a given formulation alters both the product’s environmental fate and its toxicity to different organisms.[14]
Glyphosate kills herbaceous plants through a complex, indirect mechanism. It interferes with the synthesis of certain amino acids via a biochemical pathway called the “shikimate pathway.”[15] These amino acids drive the plant’s response to a wide array of viral and bacterial pathogens, so that when a glyphosate-treated plant stops producing these amino acids, opportunistic pathogens run amok and kill the now-defenseless plants.
Glyphosate safety
When glyphosate was first introduced to the market in the 1970s, scientists generally believed that the herbicide would not be toxic to mammals since they do not have this biochemical pathway. The shikimate pathway is central to survival for plants, but not people, so it seemed plausible at the time that human risks would be minimal.
But in recent years it has become clear that bacteria also depend on the shikimate pathway.
The role of gut bacteria
Recent science has changed how both scientists and doctors view the importance of bacteria in sustaining good health in humans. For example, we now know that human bodies contain more than 10 times more bacterial cells than human cells. These bacteria are involved in many biological functions in our bodies including immunity, intestinal repair, detoxification, and production of many vitamins.
Could disruption of intestinal bacteria play a role in the rise of certain childhood illnesses?
Scientific studies indicate that gut bacteria play a central role in immunity,[16] obesity,[17] diabetes,[18] asthma,[19] food allergies,[20] inflammatory bowel disease,[21] mental health,[22] and behavior.[23]
Autistic spectrum disorder warrants special consideration. Many authors argue that the number of cases is truly on the rise,[24] and not simply a result of increasing recognition and diagnosis. The links between behavioral disturbances and changes in human gut bacterial populations are compelling, and most autistic children have serious gastrointestinal disturbances that correlate with their behavioral issues.[25] This is why many scientists see an urgent need for careful research on the impacts of glyphosate exposures on intestinal disruption and neurocognitive changes.
One study suggests that glyphosate might play a disruptive role in the intestines of certain farm animals.[26] Although it was carried out using large doses of glyphosate in petri dishes, not in actual animals, it does add to the concerns regarding the safety of glyphosate in our food supply.
Transgenic material
GE food manufacturing ingredients and animal feeds are ubiquitous in the US food supply. However, they may not contribute significant quantities of GE proteins because food manufacturing processes used to convert corn kernels, soybean seeds and canola seeds into usable products typically start to break down the GE proteins in the raw commodities.
Let’s take the example of corn: the most common type of corn grown by farmers – US #2 yellow field corn – usually contains intact, GE, protein-based Bacillus thuringiensis (Bt) toxins in the kernels at the time a field is harvested. However, the vast majority of that crop is not ingested directly by consumers: 40% is used to make ethanol, and another 40% (or slightly less) is fed to animals. Most of the remaining 20% is extensively processed. Food processing and animal digestive systems typically break down the Bt toxins into smaller fragments, about which, admittedly, relatively little is known.
For these reasons, US consumers have actually not been ingesting significant quantities of GE proteins in their intact forms. Even this, however, is beginning to change. Sweetcorn engineered to express two Bt toxins and resist glyphosate herbicide has been on the market for two years. An active 12-year old at a summer picnic who eats two ears of this Bt sweetcorn will ingest a far higher dose of relatively intact Bt toxins than ever before.
Are these foods safe?
Most feeding studies on which US regulatory agencies base their assessment that GE foods are safe were conducted for 2 or 3 months on rats.[27] The Food and Drug Administration (FDA) does not require any studies, and regulatory “consultations” are voluntary and focused simply on whether a new GE food is “substantially equivalent” in terms of composition and nutrient content to its non-engineered “isoline.” (The isoline of a GE corn, for instance, is the same variety of that corn before it was genetically altered using transgenic techniques.)
A series of reports dating back to the 1990s issued by the National Academy of Sciences called upon the FDA and other government agencies, as well as the biotechnology and food industries to develop more sensitive testing protocols in order to detect the possibly adverse impacts of subtle changes in the proteins produced in GE plants. Applying the data requirements and risk assessment tools used to test herbicides and food additives to GE foods makes about as much sense as officiating an NFL football game with the rule book governing a chess tournament.
Scientists (even within the FDA[28]) have called for longer-term studies to be performed and have expressed concern that the American public is basically now functioning as one large “experimental group.” Most of the half-dozen, well-designed long-term, GE-food feeding studies published in respected, peer-reviewed journals have reported at least some worrisome findings, often involving adverse impacts on the liver, kidneys, or lymphatic system. Despite these findings, there have been few follow-up studies, and the list of lingering concerns has grown steadily over the past decade.[29],[30],[31]
Concerns over the possible impact of GE protein-breakdown products in food, animal feed, and our bodies have grown more pressing as a result of the trend in the biotechnology seed industry to “stack” more than one GE trait in a given commercial variety. For example, most GE corn seed sold today expresses at least three traits (usually two Bts and one for glyphosate resistance), with one popular GE corn called SmartStax expressing eight (six Bts and two for herbicide tolerance). The serious concerns that persist over the safety of all individual GE crop traits now on the market become even more pressing when considering our lack of knowledge regarding the ways in which the multiple GE traits and proteins in GE corn behave and interact.
The FDA has adopted the position that if each GE-corn trait produces corn that is “substantially equivalent,” and presumably safe, then any combination of any number of previously reviewed traits must also be safe. For a science-based agency with 60 years’ experience studying drug interactions and issuing warnings about them, to simply assume that such interactions cannot occur with multiple GE traits and proteins in GE corn is an enormous leap of faith.
Conclusion
There has been too little research on the effects of foods from GE organisms on children’s health. So far, the possibility that glyphosate affects health by disrupting gut bacteria has been studied in chickens, with worrisome results,[32] but not in human children. Recently approved GE foods will expose consumers to a larger quantity of transgenic proteins. As a society, we owe it to our kids to carry out a more methodical, careful approach to introducing elements into their diets that could have adverse effects on their health and safety.
© 2015 GMO Science. All Rights Reserved
References
- Bethell CD, Kogan MD, Strickland BB, Schor EL, Robertson J, Newacheck PW. 2011. A national and state profile of leading health problems and health care quality for US children: key insurance disparities and across-state variations. Acad Pediatr. May-Jun;11(3 Suppl):S22-33. ↑
- Linabery AM, Ross JA. 2008. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. Jan 15;112(2):416-32. ↑
- Radhakrishnan DK, Dell SD, Guttmann A, Shariff SZ, Liu K, To T. 2014. Trends in the age of diagnosis of childhood asthma. J Allergy Clin Immunol. Nov;134(5):1057-62.e5. ↑
- Devereux G. 2006. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. Nov;6(11):869-74. ↑
- Lipman TH, Levitt Katz LE, Ratcliffe SJ, Murphy KM, Aguilar A, Rezvani I, Howe CJ, Fadia S, Suarez E. 2013. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. Jun;36(6):1597-603. ↑
- Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. 2010. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. Jan;50(1):27-31. ↑
- Halfon N, Houtrow A, Larson K, Newacheck PW. 2012. The changing landscape of disability in childhood. Future Child. Spring;22(1):13-42. ↑
- Hertz-Picciotto I, Delwiche L. 2009. The rise in autism and the role of age at diagnosis. Epidemiology. Jan;20(1):84-90. ↑
- Grocery Manufacturers’ Association position on GMOs: http://factsaboutgmos.org/disclosure-statement. ↑
- Agodi A, Barchitta M, Grillo A, Sciacca S. 2006. Detection of genetically modified DNA sequences in milk from the Italian market. Int J Hyg Environ Health. Jan;209:81-8. ↑
- USDA Economic Research Service. 2015. Adoption of genetically engineered crops in the United States, 1996-2015, Recent trends in GE adoption. http://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx#.VDljjLvVLRc. ↑
- Grube A DD, Kiely T, and Wu L. : Pesticides Industry Sales and Usage, 2006 and 2007 Market Estimates, United States Environmental Protection Agency, EPA 733-R-11-001, 34 p. Access at: http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf 2011. ↑
- Service. USDoANAS: Agricultural Chemical Usage – Field Crops and Potatoes. Available: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1560 (multiple years). ↑
- Mesnage R, Bernay B, Séralini GE. 2013. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. Nov 16; 313(2-3):122-8. ↑
- Duke SO, Powles SB. 2008. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci. Apr;64(4):319-25. ↑
- Cho I, Blaser MJ. 2012. The human microbiome: at the interface of health and disease. Nat Rev Genet. Mar 13;13(4):260-70. ↑
- Zhao L. 2013. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol, Sep;11(9):639-647. ↑
- Endesfelder D, zu Castell W, Ardissone A, Davis-Richardson AG, Achenbach P, Hagen M, Pflueger M, Gano KA, Fagen JR, Drew JC, Brown CT, Kolaczkowski B, Atkinson M, Schatz D, Bonifacio E, Triplett EW, Ziegler AG. 2014. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes. Jun;63(6):2006-14. ↑
- See reference 3 above ↑
- Round JL and Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. May; 9(5):313-23. ↑
- Kostic AD, Xavier RJ, Gevers D. 2014. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. May;146(6):1489-99. ↑
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. Dec 19;155(7):1451-63. ↑
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. Dec 19;155(7):1451-63. ↑
- Hertz-Picciotto I, Delwiche L. 2009. The rise in autism and the role of age at diagnosis. Epidemiology. Jan;20(1):84-90. ↑
- Mulle JG, Sharp WG, Cubells JF. 2013. The Gut Microbiome: A New Frontier in Autism Research. Curr Psychiatry Rep. Feb;15(2): 337. ↑
- Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M. 2013. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol. Apr;66(4):350-8. ↑
- Domingo JL, Giné Bordonaba J. 2011. A literature review on the safety assessment of genetically modified plants. Environ Int. May;37(4):734-42. ↑
- Louis J. Pribyl, Ph.D., scientist in the FDA’s Microbiology Group in a February 1992 memo, “There is a profound difference between the types of unexpected effects from traditional breeding and genetic engineering which is just glanced over in this document…”Linda Kahl, Ph.D., FDA compliance officer, January, 1992 memo, “There is no data that could quantify risk” (regarding GE crops).
E.J. Matthews, Ph.D., FDA Toxicology group, October 1991 memo, “genetically modified plants could also contain unexpected high concentrations of plant toxicants.”
Quoted in Roseboro K. 2011. FDA ignored own scientists’ warnings about GM foods, http://www.nongmoreport.com/articles/october2011/FDAignoredscientistswarningsGMfoods.php#sthash.ErX2vAdm.dpuf ↑ - Netherwood T, Martin-Orue SM, O’Donnell AG, Gockling S, Graham J, Mathers JC, Gilbert HJ. 2004. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 22(2):204-9. ↑
- Mesnage R, Arno M, Costanzo M, Malatesta M, Séralini GE, Antoniou MN. 2015. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ Health. 2015 Aug 25;14(1):70. ↑
- Mesnage R, Defarge N, Spiroux de Vendômois J, Séralini GE. 2015. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol. [Epub ahead of print] ↑
- Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M. 2013. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol. Apr;66(4):350-8. ↑
Related Posts
OverSanitized
The Clean Hand Obsession From supermarkets to schoolyards, it is hard to travel more than ten feet without running into a bottle of hand sanitizer! ...
An Indigenous Woman Fights Back Against Monsanto and Wins
One person can make a difference! A 55-year-old native Mayan beekeeper, Leydy Pech, has won out against Monsanto in a grassroots effort to protect...
You Asked! We Answered! Do Carbon Filters Eliminate Glyphosate?
Reader Kathryn L. asks whether carbon filters can eliminate glyphosate from her drinking water. Good question! This is what we found out As per...